- Home
- Industries
- Life Science
Achieve Consistent Results Across Your Operations
Cybertrol Engineering is your trusted partner in control and information systems integration for the highly-regulated life science industry. With years of process control experience and a commitment to excellence, we specialize in delivering tailored solutions to medical device, pharmaceutical, biotechnology, and nutraceutical manufacturers, enabling them to achieve unparalleled levels of quality, validation, tracking, and traceability throughout their operations.
Our Expertise in the Life Science Industry
Cybertrol Engineering helps manufacturers in life sciences enhance their operational agility and market responsiveness through our plantwide automation and information solutions. Our expertise in automated process control and real-time production data access accelerates time to market, while simultaneously simplifying compliance procedures and minimizing risks. Plus, our comprehensive integration solutions facilitate seamless collaboration across various business functions, unlocking opportunities for innovation.
A well-designed digital transformation architecture with interoperability and security features is essential for any Life Science organization aspiring to elevate their business performance. With Cybertrol Engineering as your partner, you can confidently navigate the complexities of digital transformation and embark on a journey towards unparalleled success in the industry.
Life Science Industry Expertise
- Aseptic Filling
- Batch Process Control Systems – Recipe Driven Manufacturing/ISA-88 Standards
- Batch Weighing and Loading
- Building and Environmental Management Systems (BMS/EMS)
- CIP (Clean-in-place)/SIP (Sterilize-in-place)
- Conveyor Controls
- Coordinated Drive Systems
- Electronic Batch Records (EBR)
- Fermentation
- Fill-Finish
- Freeze Dryers
- Inventory management
- Lyophilization
- Packaging and Assembly
- Parts Identification and Tracking
Consistent Quality and Compliance
Cybertrol has a proven track record of collaborating with the top manufacturing companies in the life science sector. We understand the unique challenges and stringent requirements of this industry, and our team of experts is dedicated to delivering solutions that are designed to ensure adherence to industry standards and regulations such as FDA 21 CFR Part 11 (Code of Federal Regulations for electronic records and electronic signatures), allowing our clients to maintain the highest levels of quality and compliance across their operations.
Comprehensive Support Across the System Development Life Cycle
From initial concept to final validation and ongoing optimization, Cybertrol is with you every step of the way. We excel in leading projects through the system development life cycle, starting with comprehensive user and functional requirements gathering and continuing through qualification and validation planning. Our expertise in all aspects of GAMP (Good Automated Manufacturing Practice) ensures that your projects are executed with precision and efficiency, saving you time and resources while maximizing results.
The Cybertrol Difference
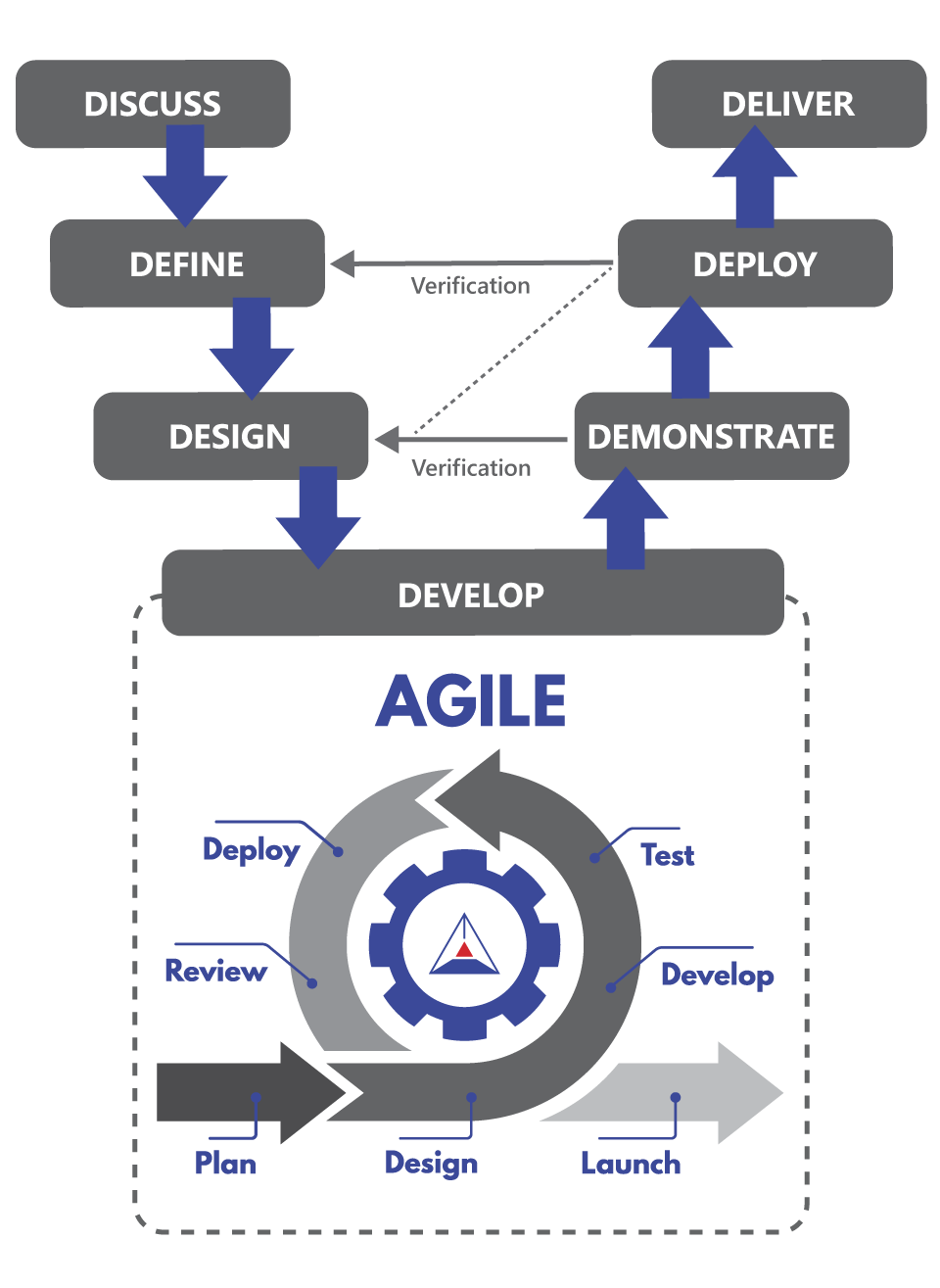
Our Proven Process
Cybertrol uses a customer-centric approach to implement projects. We engage with experts within our customers' facilities and partner design firms, leveraging our experience to create a unified plant operation.
DISCUSS
User Requirement Specifications:
Cybertrol will engage in discussions with the customer to understand their primary pain points and collaboratively develop the user requirement specifications.
Deliverables:
- Definition
DEFINE
Functional Definition:
In this phase, Cybertrol develops the functional definitions and creates the system and software architectural drawings. These are then reviewed with the customer and the design team.
Deliverables:
- Functional descriptions
- System architecture documents
DESIGN
Design Specifications:
In the design phase, detailed requirements are provided to the Cybertrol team. These requirements guide the development of the specific documents needed for the system build process.
Deliverables:
- Software design specifications
- Electrical drawings
DEVELOP
System Build:
Cybertrol employs the Agile methodology by clearly defining the criteria for "done" and utilizing an iterative development cycle. This approach ensures customer expectations are met and development time is reduced.
Deliverables:
- PLC programming
- HMI programming
- SCADA development
- Industrial control panels
DEMONSTRATE
Installation Qualification/SAT (IQ):
During the demonstrate phase, which occurs throughout the Agile development stage, a Software Acceptance Test (SAT) is conducted. This test runs through the entire process and reviews the panel builds before they are implemented at the plant.
Deliverables:
- Software verification
- Drawing verification
- PLC IO verification
- Hardware verification
- Network address verification
DEPLOY
Operational Qualification/IO Testing and Functional Testing (OQ):
Cybertrol collaborates with the implementation team to conduct functional testing of all devices, run sequencing through the installed equipment without product, and perform trial operations. This ensures the system operates according to the design specifications.
Deliverables:
- Function testing
- HMI – Screen, parameter, and security testing
- Alarm testing
- Report verification
- SCADA functionality verification
DELIVER
Performance Qualification (PQ):
This final stage involves production testing and validation of the system to ensure it meets performance standards before handing it over to the end user.
Deliverables:
- Final testing
- System hand over
Seamless Integration Across Control Layers
Our comprehensive suite of information solutions seamlessly integrates with control layers, providing a unified platform for data collection, management, and analysis. From process control to data visualization, Cybertrol's solutions enable you to optimize your operations and make informed decisions in real time.
Configurable Software Solutions and Core Programming Modules
We understand that every project and every system is unique. That's why we offer configurable software solutions (BatchWorks MES and Configurable CIP) and proven core programming modules that can be tailored to meet the specific needs of each client. Whether you're looking for a complete control system solution or individual modules to enhance your existing infrastructure, Cybertrol has you covered.

Plantwide Capabilities in the Life Sciences Industry
Cybertrol's extensive capabilities in the life sciences industry encompass every stage of the system development life cycle. From start to finish, our team is equipped to guide your projects seamlessly through each phase:
We work closely with you to understand your unique needs and requirements, ensuring our solutions align perfectly with your objectives.
Our team of engineers will translate user requirements into comprehensive functional specifications, laying the groundwork for a successful project.
With meticulous attention to detail, we design and build custom systems tailored to your exact specifications, guaranteeing optimal performance and functionality.
Prior to implementation, our offline software testing procedures ensure reliability and accuracy, mitigating risks and ensuring smooth operation.
Our thorough installation qualification processes verify that systems are installed correctly and meet all necessary specifications and regulations.
We validate system operations to ensure they function as intended, providing assurance of performance and reliability.
Through rigorous performance qualification testing, we confirm that systems meet predefined criteria and deliver consistent results.
Testimonials
Contact us to discover how you can achieve consistent quality, validation, tracking, and traceability across your operations.
Contact Us




